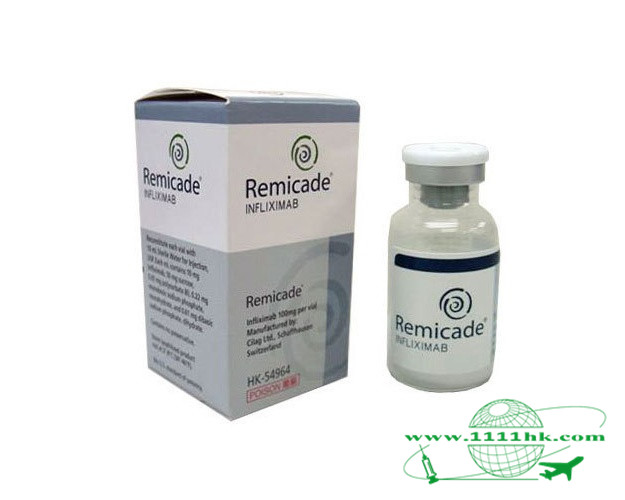
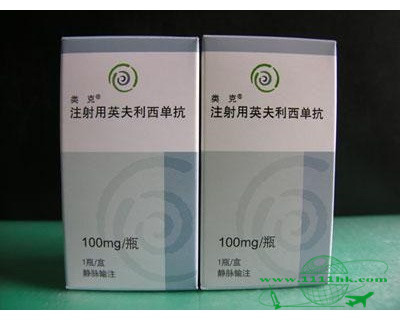
英夫利昔单抗
【商品名】REMICADE, Remicade
【英文通用名】Infliximab
【中文通用名】英夫利昔单抗
【英文其它名】
【中文其它名】
【适应症】
类风湿关节炎
本品是疾病控制性抗风湿药物。对于中重度活动性类风湿关节炎患者,本品与甲氨蝶呤合用可用于:
·减轻症状和体征;
·改善身体机能,预防患者残疾。
克罗恩病
对于接受传统治疗效果不佳的中重度活动性克罗恩病患者,本品可用于:
·减轻症状和体征;
·达到并维持临床疗效;
·促进粘膜愈合;
·改善生活质量;
·使患者减少皮质激素用量或停止使用皮质激素。
瘘管性克罗恩病
对于瘘管性克罗恩病患者,本品可用于:
·减少肠-皮肤瘘管和直肠-阴道瘘管的数量,促进并维持瘘管愈合;
·减轻症状和体征;
·改善生活质量。
强直性脊柱炎
对于活动性强直性脊柱炎患者,本品可用于:
·减轻症状和体征,包括增加活动幅度;
·改善身体机能;
·改善生活质量。
银屑病
对于银屑病患者,本品可用于:
需系统治疗且对环孢霉素、甲氨蝶呤或光化学疗法等其它系统治疗无效、禁忌或耐受的慢性重度斑块型银屑病成年患者。本品仅用于能在医师的密切监测下进行治疗并由医生进行定期随访的患者。
【用法用量】
用法:静脉输注。
类风湿关节炎
首次给予本品3mg/kg,然后在首次给药后的第2周和第6周及以后每隔8周各给予一次相同剂量。本品应与甲氨蝶吟合用。对于疗效不佳的患者,可考虑将剂量调整至10mg/kg,和/或将用药间隔调整为4周。
中重度活动性克罗恩病、瘘管性克罗恩病
首次给予本品5mg/kg,然后在首次给药后的第2周和第6周及以后每隔8周各给予一次相同剂量。对于疗效不佳的患者,可考虑将剂量调整至l0mg/kg。
强直性脊柱炎
首次给予本品5mg/kg,然后在首次给药后的第2周和第6周及以后每隔6周各给予一次相同剂量。
使用指导
应进行无菌操作。
1.计算剂量,确定本品的使用瓶数:本品每瓶含英夫利西单抗100mg,计算所需配制的本品溶液总量。
2.使用配有21号(0.8mm)或更小针头的注射器,将每瓶药品用10ml无菌注射用水溶解:除去药瓶的翻盖,用医用酒精棉签擦拭药瓶顶部,将注射器针头插入药瓶胶盖,注入无菌注射用水。如药瓶内的真空状态已被破坏,则该瓶药品不能使用。轻轻旋转药瓶,使药粉溶解。避免长时间或用力摇晃,严禁振荡。溶药过程中可能出现泡沫,放置5分钟后,溶液应为无色或淡黄色,泛乳白色光。由于英夫利西单抗是一种蛋白质,溶液中可能会有一些半透明微粒。如果溶液中出现不透明颗粒、变色或其它物质,则不能继续使用。
3.用0.9%氯化钠注射液将本品的无菌注射用水溶液稀释至250ml:从250ml 0.9%氯化钠注射液瓶或袋中抽出与本品的无菌注射用水溶液相同的液体量,将本品的无菌注射用水溶液全部注入该输液瓶或袋中,轻轻混合。
4.输液时间不得少于2小时:输液装置上应配有一个内置的、无菌、无热原、低蛋白结合率的滤膜(孔径≤1.2μm)。未用完的输液不应再贮存使用。
5.未进行本品与其它药物合用的物理生化兼容性研究,本品不应与其它药物同时进行输液。
经胃肠道外给药的产品在给药前应目检是否存在微粒物质或变色现象。如果发现存在不透明颗粒、变色或其它异物,则该药品不可使用。
斑块型银屑病
首次给予本品5mg/kg,然后在首次给药后的第2周和第6周及以后每隔8周各给予一次相同剂量。若患者在第14周后(即4次给药后)没有应答,不应继续给予本品治疗。
银屑病患者再次给药
银屑病患者相隔20周后再次单次给药的经验有限,与最初的诱导治疗相比,提示本品的有效性降低,且轻到中度输液反应增加。
疾病复发后,有限的反复诱导治疗经验表明,与8周维持治疗相比,输液反应增加(包括严重反应)。
如维持治疗中断,不推荐再次启动诱导治疗,应按照维持治疗再次给药。
【贮藏】2-8℃避光保存。
【温馨提示】本站部分信息来源于互联网,仅为药师或医护人员内部讨论使用,不能代替医生当面诊断。具体用药事宜请咨询专业药剂师,产品内容请以实际产品说明书或实物为准。