托珠单抗
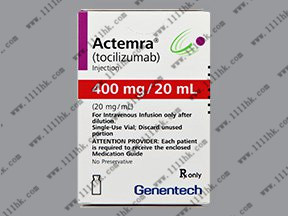
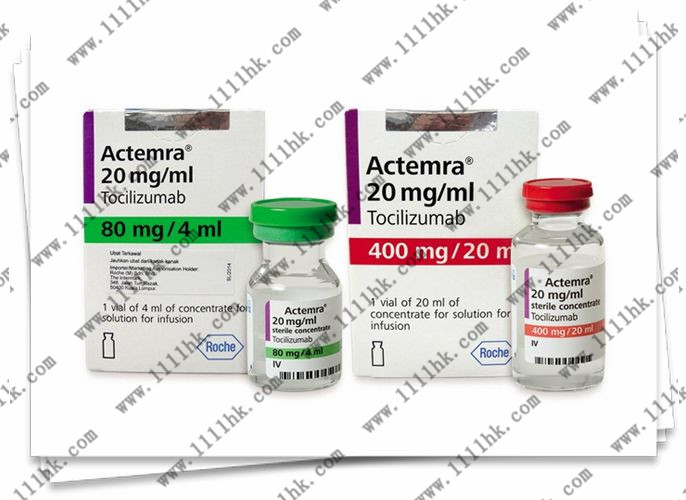
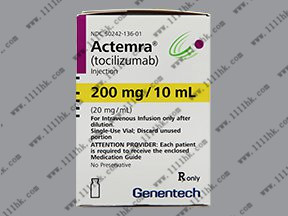
【商品名】ACTEMRA
【英文通用名】TocilizumabInjection
【中文通用名】托珠单抗注射液
【英文其它名】tocilizumab
【中文其它名】
【适应症】适用于一种或更多TNF 拮抗剂治疗反应不佳的中度至严重-活动性类风湿性关节炎成年患者。
【用法用量】类风湿性关节炎。ACTEMRA可单独使用或与氨甲喋呤或其它DMARD联用。
1. 成年推荐剂量每4周:患者对一种或更多TNF拮抗剂反应不佳:当与DMARD联用或单药治疗时,推荐起始量是4 mg/kg接着基于临床反应增至8 mg/kg。嗜中性绝对计数(ANC)低于2000/mm3, 血小板计数低于100,000/mm3,或ALT或AST高于正常上限(ULN)1.5倍患者建议不要开始用ACTEMRA。
2. 建议每次输注ACTEMRA剂量不要超过800mg。
3. 给药为静脉输注用无菌术稀释至100mL 0.9%氯化钠。在1小时期间单次静脉滴注。不要推注。
4. 调整剂量 建议对某些剂量-相关实验室变化处理包括肝酶升高、白细胞减少、和血小板减少。
【贮藏】存放在冰箱中(2°C-8°C)。 不要冻结。 将预先装好的注射器放在外纸箱中,以防止光线和湿气。
【温馨提示】本站部分信息来源于互联网,仅为药师或医护人员内部讨论使用,不能代替医生当面诊断。具体用药事宜请咨询专业药剂师,产品内容请以实际产品说明书或实物为准。
名称
Attention: The internal data of table “640” is corrupted!
产品
Attention: The internal data of table “641” is corrupted!