奥希替尼
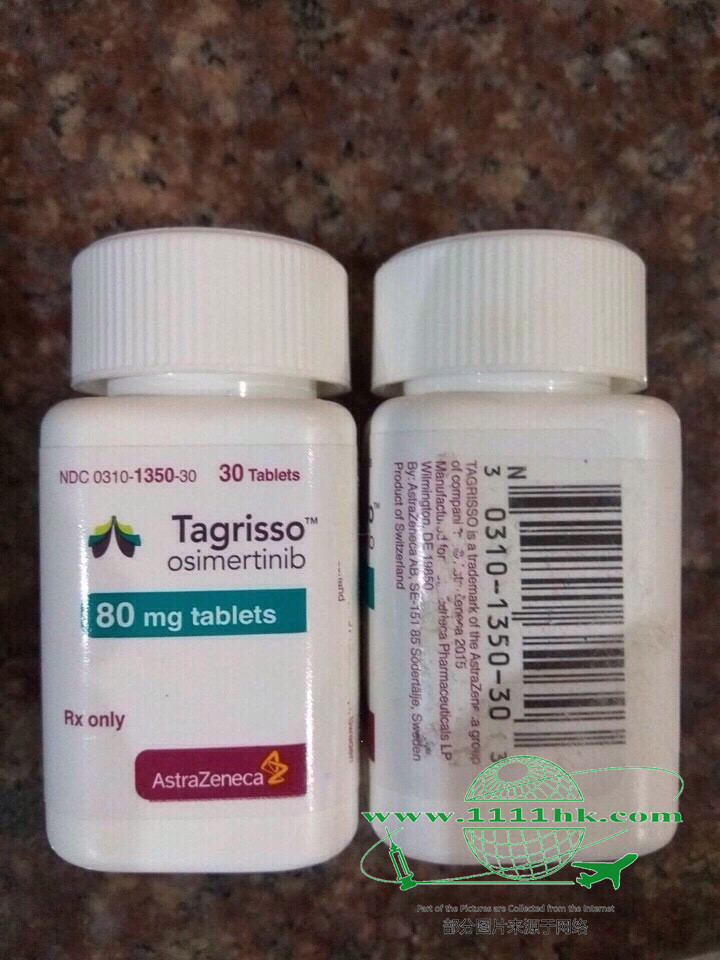
【商品名】Tagrisso
【英文通用名】Ochinib
【中文通用名】奥希替尼
【英文其它名】
【中文其它名】
【适应症】本品适用于既往经表皮生长因子受体(EGFR)酪氨酸激酶抑制剂(TKI)治疗时或治疗后出现疾病进展,并且经检测确认存在EGFR T790M突变阳性的局部晚期或转移性非小细胞性肺癌(NSCLC)成人患者的治疗。
【用法用量】本品应由在抗肿瘤治疗方面富有经验的医生处方使用。
在使用本品治疗局部晚期或转移性NSCLC前,首先需要明确EGFR T790M突变的状态。应采用经过充分验证的检测方法确定存在EGFR T790M突变方可使用本品治疗。
剂量
本品的推荐剂量为每日80mg,直至疾病进展或出现无法耐受的毒性。
如果漏服本品1次,则应补服本品,除非下次服药时间在12小时以内。
本品应在每日相同的时间服用,进餐或空腹时服用均可。
剂量调整
根据患者个体的安全性和耐受性,可暂停用药或减量。如果需要减量,则剂量应减至40mg,每日1次。
特殊人群
无需因为患者的年龄、体重、性别、种族和吸烟状态对剂量进行调整。
肝功能损害
轻度肝功能损害(总胆红素<正常值上限(ULN)且谷草转氨酶(AST)达1至1.5xULN;或总胆红素达1至1.5xULN,AST不限)患者无需进行剂量调整,但此类患者仍应慎用本品。中重度肝功能损害患者使用本品的安全性和有效性尚不明确。在获得更多信息前,不建议中重度肝功能损害患者使用本品。 (见[药代动力学])。
肾功能损害
轻中度肾功能损害患者使用本品时无需进行剂量调整。重度肾功能损害患者使用本品的数据有限。终末期肾病(经Cockcroft 和 Gault方程计算的肌酐清除率(CLcr)<15mL/min)或正在接受透析的患者使用本品的安全性和有效性尚不明确。患有重度或终末期肾功能损害的患者应慎用本品 (见[药代动力学])。
给药方法
本品为口服使用。本品应整片和水送服,不应压碎、掰断或咀嚼。
如果患者无法吞咽药物,则可将药片溶于50mL不含碳酸盐的水中。应将药片投入水中,无需压碎,直接搅拌至分散后迅速吞服。随后应再加入半杯水,以保证杯内无残留,随后迅速饮用。不应添加其它液体。
需要经胃管喂饲时,可采用和上述相同的方式进行处理,只是最初溶解药物时用水15mL,后续残余物冲洗时用水15mL。这30mL液体均应按鼻胃管生产商的说明进行喂饲,同时用适量的水冲洗。这些溶解液和残余液均应在将药片加入水中后30分钟内服用。
【贮藏】30℃以下保存。
【温馨提示】本站部分信息来源于互联网,仅为药师或医护人员内部讨论使用,不能代替医生当面诊断。具体用药事宜请咨询专业药剂师,产品内容请以实际产品说明书或实物为准。