Montelukast sodium
【Product name】KIPRES, MONTELUKAST, SINGULAIR
[English common name]Montelukast Sodium
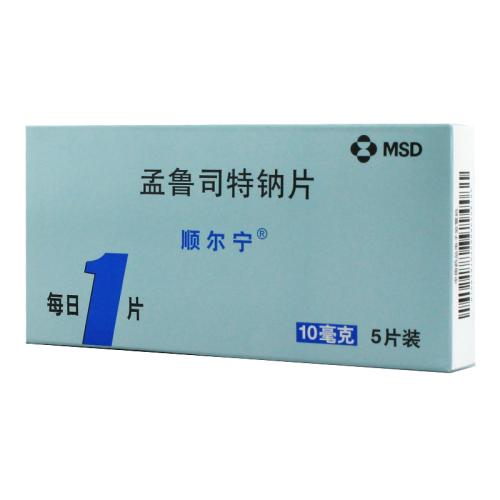
[Chinese common name]Montelukast sodium
[English name]
[Chinese other name]
[indications]
Montelukast sodium tablets: This product is suitable for adults aged 15 and over.asthmaPrevention and long-term treatment, including prevention of asthma symptoms during the day and night, treatment of asthma patients who are sensitive to aspirin, and prevention of exercise-induced bronchoconstriction. This product is suitable for alleviating the symptoms caused by allergic rhinitis (seasonal allergic rhinitis and perennial allergic rhinitis in adults aged 15 and over).
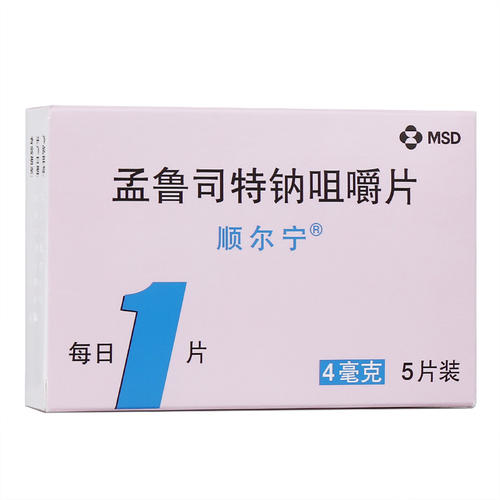
Montelukast sodium chewable tablets: This product is suitable for the prevention and long-term treatment of asthma in children aged 2 to 14 years, including prevention of asthma symptoms during the day and night, treatment of aspirin-sensitive asthma patients and prevention of exercise-induced bronchoconstriction. This product is suitable for alleviating the symptoms caused by allergic rhinitis (seasonal allergic rhinitis and perennial allergic rhinitis in children aged 2 to 14 years).
Montelukast sodium granules: This product is suitable for the prevention and long-term treatment of asthma in children over one year old, including prevention of asthma symptoms during the day and night, treatment of aspirin-sensitive asthma patients and prevention of exercise-induced bronchoconstriction. This product is suitable for alleviating the symptoms caused by allergic rhinitis (seasonal allergic rhinitis and perennial allergic rhinitis in children aged 2 to 5 years old).
【Dosage】
Montelukast sodium tablets: once a day, one tablet each time (10 mg). Asthmatic patients should take it before going to bed. Patients with allergic rhinitis can take it when needed according to their own conditions.
Patients with both asthma and allergic rhinitis should be given once a night. Adult patients with asthma and/or allergic rhinitis 15 years and older are administered once daily for 10 mg each time.
General advice
The therapeutic effect was evaluated by the asthma control index, and the efficacy of the product appeared within one day of the drug administration. This product can be taken with food or served separately. Patients should be advised to take it regardless of asthma control or deterioration.
Older patients, patients with renal insufficiency, patients with mild to moderate liver damage, and patients of different genders do not need to adjust the dose.
Relationship between montelukast sodium tablets and other asthma treatment drugs
This product can be added to the patient's existing treatment plan.
Reduce the dose of the combined medication:
Bronchodilator
Asthma patients who can not effectively control bronchodilator alone can be added to the treatment plan. Once there is obvious clinical response (usually after the first dose), the bronchodilator can be used according to the patient's tolerance. The dose is reduced.
Inhaled corticosteroids
For patients with asthma who receive inhaled corticosteroids, the dose of glucocorticoids can be appropriately reduced according to the patient's tolerance. It should be gradually reduced under the guidance of a physician. Some patients can gradually reduce the amount until inhaled glucocorticoids are completely discontinued. However, this product should not be used to suddenly replace inhaled corticosteroids.
Montelukast sodium chewable tablets: once daily. Asthmatic patients should take it before going to bed. Patients with allergic rhinitis can take the medicine according to their own circumstances.
Patients with both asthma and allergic rhinitis should be given once a night.
Children aged 6 to 14 years with asthma and / or allergic rhinitis once a day, one tablet at a time (5mg).
Children from 2 to 5 years old with asthma and/or allergic rhinitis once daily, one tablet at a time (4 mg).
General advice
The therapeutic effect was evaluated by the asthma control index, and the efficacy of the product appeared within one day of the drug administration. This product can be taken with food or served separately. Patients should be advised to take it regardless of asthma control or deterioration.
There is no need to adjust the dose for patients with renal insufficiency, patients with mild to moderate liver damage, and patients of different genders.
The relationship between this product and other asthma treatment drugs
This product can be added to the patient's existing treatment plan.
Reduce the dose of the combined drug:
Bronchodilator
In asthma patients who cannot be effectively controlled with bronchodilator alone, this product can be added to the treatment regimen. Once there is a clinical response (usually after the first dose), the dose of bronchodilator can be reduced according to the patient's tolerance. .
Inhaled corticosteroids
For patients with asthma who receive inhaled corticosteroids, the dose of glucocorticoids can be appropriately reduced according to the patient's tolerance. It should be gradually reduced under the guidance of a physician. Some patients can gradually reduce the amount until inhaled glucocorticoids are completely discontinued. However, this product should not be used to suddenly replace inhaled corticosteroids or as directed by your doctor.
Montelukast sodium granules: once daily. Asthmatic patients should take it before going to bed. Patients with allergic rhinitis can take the medicine according to their own circumstances.
Patients with both asthma and allergic rhinitis should be given once a night.
Asthma patients from 1 to 2 years old once a day, one bag at a time.
Asthma patients aged 2 to 5 years and/or patients with allergic rhinitis between the ages of 2 and 5 years.
Take 4mg of oral granules a day.
Oral granules
This product can be taken directly, mixed with a spoonful of soft or soft food (such as applesauce), or dissolved in a teaspoon of room temperature or cold infant formula or breast milk. The bag can only be opened when taken. Take all doses (within 15 minutes) immediately after opening the bag. This product, mixed with food, infant formula or breast milk, can no longer be stored until the next time. This product should not be dissolved in other liquids other than infant formula or breast milk. However, you can drink water after taking the medicine.
General advice
The therapeutic effect was evaluated by the asthma control index, and the efficacy of the product appeared within one day of the drug administration. This product can be taken with food or served separately. Patients should be advised to take it regardless of asthma control or deterioration.
There is no need to adjust the dose for patients with renal insufficiency, patients with mild to moderate liver damage, and patients of different genders.
The relationship between this product and other asthma treatment drugs
This product can be added to the patient's existing treatment plan.
Reduce the dose of the combined drug:
Bronchodilator
In asthma patients who cannot be effectively controlled with bronchodilator alone, this product can be added to the treatment regimen. Once there is a clinical response (usually after the first dose), the dose of bronchodilator can be reduced according to the patient's tolerance. .
Inhaled corticosteroids
For patients with asthma who receive inhaled corticosteroids, the dose of glucocorticoids can be appropriately reduced according to the patient's tolerance. It should be gradually reduced under the guidance of a physician. Some patients can gradually reduce the amount until inhaled glucocorticoids are completely discontinued. However, this product should not be used to suddenly replace inhaled corticosteroids or as directed by your doctor.
[Storage]
Montelukast sodium tablets: stored at 15-30 ° C at room temperature, moisture and shading.
Montelukast sodium chewable tablets: stored at 15-30 ° C at room temperature, moisture and shading.
Montelukast sodium granules: sealed, protected from light, and stored at room temperature (15-30 ° C).
[Tips] Some of the information on this site comes from the Internet and is only used internally by pharmacists or medical staff. It is not a substitute for doctors to diagnose face to face. Please consult a professional pharmacist for specific medications. Please refer to the actual product manual or the actual product for the product content.