达拉非尼
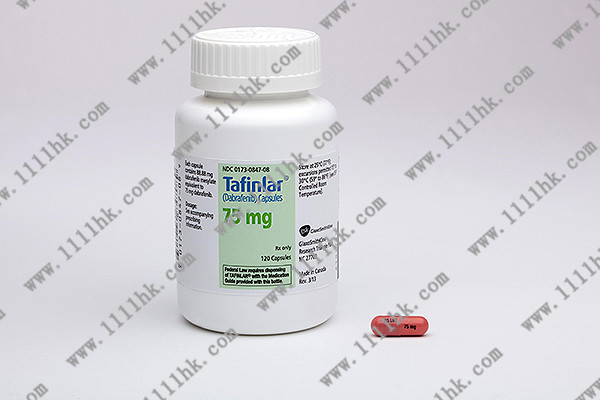
【商品名】Tafinlar
【英文通用名】Dabrafenib
【中文通用名】达拉非尼
【英文其它名】
【中文其它名】
【适应症】
1.不可切除或已经转移的BRAF V600E基因突变型黑色素瘤(不适用于BRAF野生型黑色素瘤)。
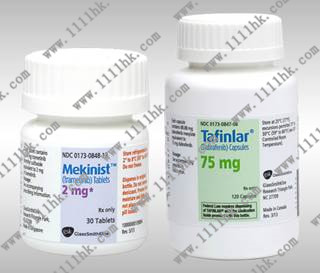
2.与曲美替尼联用适用于有BRAF V600E或V600K突变的,不可切除的或转移性黑色素瘤患者的治疗。
【用法用量】150mg,口服,每日2次(间隔12小时),持续给药直到疾病进展或出现不可耐受的毒性。
1.饭前1小时或饭后2小时服用
2. 丢失剂量可在距离下一次剂量6小时之前服用,不可在距离下一剂6小时内服用
3.不要打开,压碎,或打破胶囊服用
【贮藏】储存在25°C(77°F); 允许偏移15°C至30°C(59°F至86°F)
【温馨提示】本站部分信息来源于互联网,仅为药师或医护人员内部讨论使用,不能代替医生当面诊断。具体用药事宜请咨询专业药剂师,产品内容请以实际产品说明书或实物为准。
名称
Attention: The internal data of table “55” is corrupted!
产品
Attention: The internal data of table “56” is corrupted!