β-半乳糖苷酶A
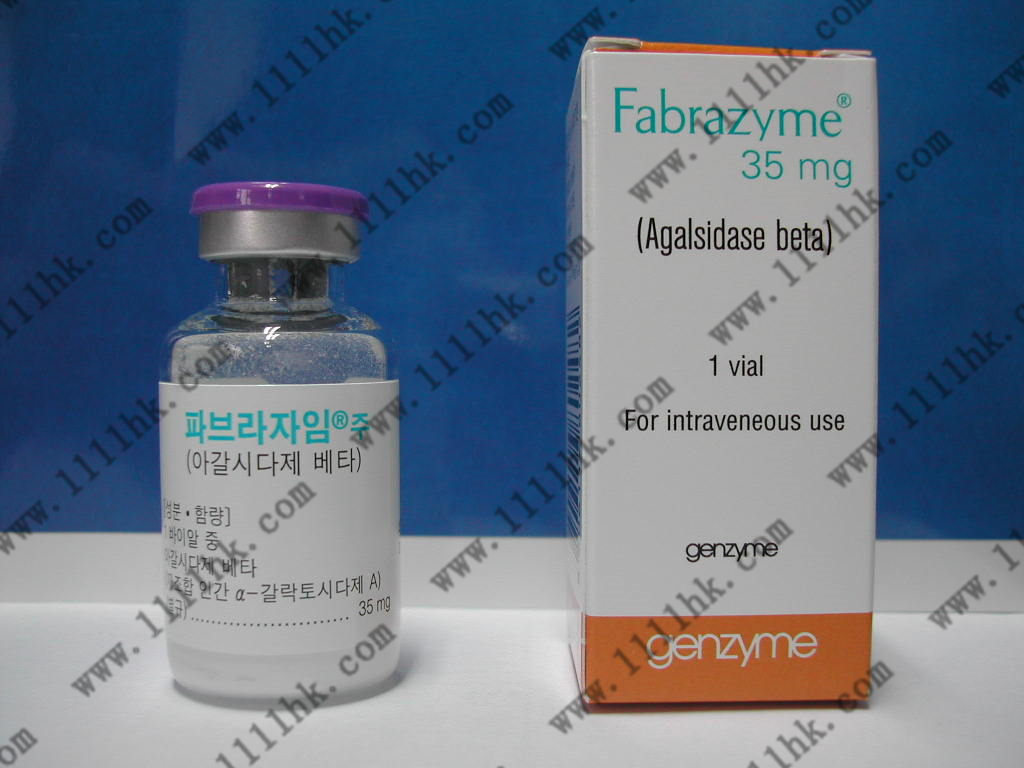
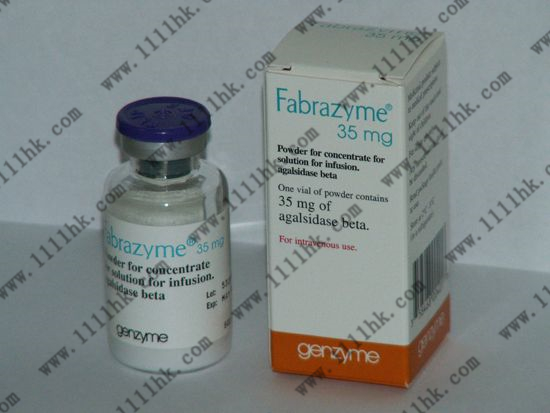
【商品名】Fabrazyme
【英文通用名】agalsidase beta
【中文通用名】半乳糖苷酶
【英文其它名】beta-Galactosidase, Lactase, Galactosidase
【中文其它名】β-半乳糖苷酶, beta-半乳糖苷酶
【适应症】Fabrazyme适用于确诊为法布里病(α-半乳糖苷酶A缺乏症)的患者的长期酶替代疗法。
Fabrazyme适用于8岁及以上的成人,儿童和青少年。
【用法用量】剂量学
Fabrazyme的推荐剂量是1mg / kg体重,每2周施用一次作为静脉内输注。
较低的给药方案已用于临床研究。在其中一项研究中,在成年男性患者中进行,在每2周初始剂量为1.0 mg / kg,持续6个月后,每2周0.3 mg / kg可在某些患者的某些细胞类型中维持GL-3的清除;然而,这些发现的长期临床相关性尚未确定。
初始输注速度应不超过0.25毫克/分钟(15毫克/小时),以尽量减少输液相关反应的可能发生。在建立患者耐受性之后,输注速率可以随后随后输注逐渐增加。
对于能够耐受其输注的患者,可考虑在家中输注Fabrazyme。
应该在评估后做出让患者转入家庭输液的决定
主治医师的推荐。患者在家中经历不良事件
输液需要立即停止输液过程并寻求医疗保健的关注专业的。随后的输注可能需要在临床环境中进行。剂量和输注率在家时应保持不变,如果没有医疗保健的监督,不得改变专业的。
特殊人群
肾功能不全
肾功能不全患者无需调整剂量。
肝功能损害
尚未对肝功能不全患者进行研究。
老年
Fabrazyme在65岁以上患者中的安全性和有效性尚未确定,也没有目前可以在这些患者中推荐给药方案。
小儿人口
Fabrazyme在0至7岁儿童中的安全性和有效性尚未确定。
目前可用的数据在5.1和5.2节中描述,但没有关于posology的建议适用于5至7岁的儿童。 0至4岁儿童无法获得数据8-16岁儿童无需调整剂量
【贮藏】存放在冰箱中(2°C – 8°C)。
【温馨提示】本站部分信息来源于互联网,仅为药师或医护人员内部讨论使用,不能代替医生当面诊断。具体用药事宜请咨询专业药剂师,产品内容请以实际产品说明书或实物为准。